Ocean Acidification
Ocean acidification is the decrease in the pH of the ocean and the subsequent changes in seawater
chemistry that are caused by the oceans absorbing increasing amounts of carbon dioxide.
Education and Outreach
On May 14, 2014, the Snohomish County MRC co-hosted a forum on ocean health with the Northwest Straits Commission and the City of Everett. The event was moderated by Snohomish County Council Chair Dave Somers. Snohomish MRC Vice-Chair Franchesca Perez opened the evening with an IGNITE talk for presenters Dr. Terrie Klinger, Simon Geerlofs, and Betsy Peabody. The forum focused on developing an understanding of the global issues of ocean acidification and sea level rise and what is being done to address them locally. Video footage of the available presentations can be viewed by clicking on the links below.
- Global Challenges and Making a Difference at the Local Scale - Simon Geerlofs
- A Multi-Faceted Approach to Combatting Ocean Acidification - Betsy Peabody
The Process of Ocean Acidification
When carbon dioxide (CO2) is released into the atmosphere, primarily through the burning of fossil fuels, about 25% of these emissions are absorbed by the ocean. The CO2 reacts with the elements dissolved in seawater to form carbonic acid, which lowers the pH of seawater. Since the Industrial Revolution, the oceans' pH has decreased by 0.1 units, which is equivalent to a 26-30% increase in the oceans’ acidity.
Increasing levels of carbonic acid, a corrosive compound, dissolve or inhibit the growth of marine organisms called calcifiers whose shells and skeletons are composed of calcium carbonate (CaCO3). Vulnerable organisms include oysters, clams, mussels, abalone, crabs, corals, and animals at the base of the marine food web such as zooplankton and pteropods.
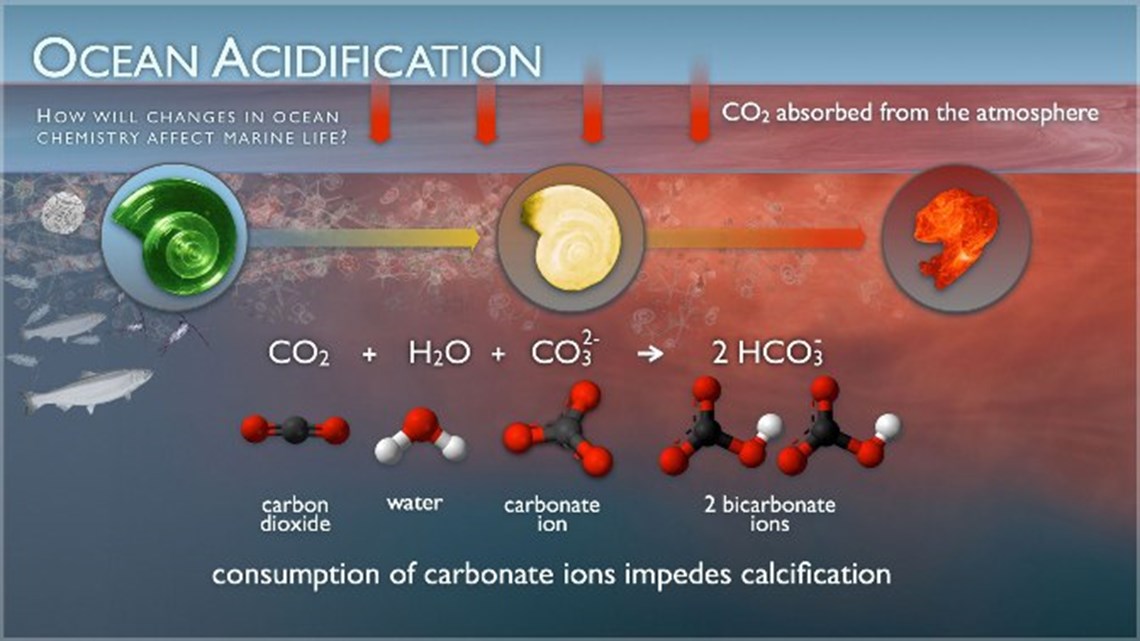
However, the effects of ocean acidification are not limited to calcifiers. Disruptions at the bottom of the food web ripple upward and outward. Direct and indirect effects will be visible throughout the entire marine ecosystem. Current research is measuring the effects of ocean acidification on a variety of species.
Pacific Northwest Impacts
Marine ecosystems are already being impacted, especially in the Pacific Northwest. The coastal upwelling on the Washington coast brings cold, nutrient-rich waters from the deep ocean to the surface. This nutrient-rich water is also rich in carbon dioxide and low in pH, exacerbating the effects of ocean acidification in the Pacific Northwest. Since 2005, the effects of ocean acidification have been observed in Washington, although they were not initially identified as such. Shellfish farmers have experienced decreases in shellfish size, abnormal growth patterns and mortality rates of up to 80% in shellfish larva.
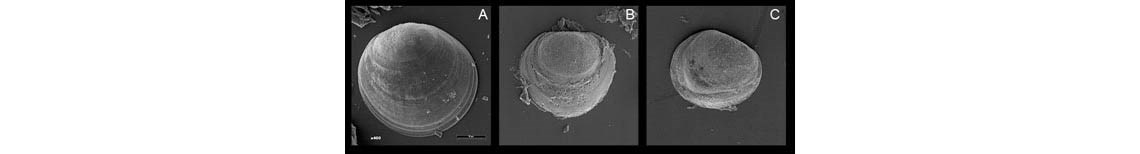
When exposed to a pH of 7.5, a common hardshell clam from the East Coast cannot survive. The first day of exposure, the larvae are healthy (A). On day 2, the shells are visibly dissolving (B). By day 3, the larvae are almost dead or dead (C).
Citations
- National Oceanic and Atmospheric Administration (NOAA) Pacific Marine Environmental Laboratory (PMEL). Ocean Acidification: The Other Carbon Dioxide Problem.
- Feely, R.A., T. Klinger, J.A. Newton, and M. Chadsey (2012). Scientific Summary of Ocean Acidification in Washington State Marine Waters. NOAA OAR Special Report.
- Woods Hole Institute (WHOI). The pH Scale.
- National Oceanic and Atmospheric Administration (NOAA) Northwest Fisheries Science Center. CO2 and Ocean Acidity.
- National Oceanic and Atmospheric Administration (NOAA). Upwelling is a process in which deep, cold water rises toward the surface.
- National Oceanic and Atmospheric Administration (NOAA), ‘Like putting headlights on a car’ Pacific oysters gain from IOOS® data
Information and Resources
- Washington Sea Grant Ocean Acidification Page
- Ocean Acidification in the Pacific Northwest
- 20 Facts about Ocean Acidification
- Ocean Acidification (University of Washington)
- Ocean Acidification: From Knowledge to Action
- NOAA Ocean Acidification Program
- Northwest Association of Networked Ocean Observing Systems (NANOOS )
- FAQs about Ocean Acidification From Woods Hole Oceanographic Institution
- Study Shows Ocean Acidification May Directly Harm Fish
Ocean Acidification Seminar Presentations
- Ocean Acidification: What is it? (PDF)
- Ocean acidification and its biological impacts (PDF)
- Biting Godzilla: How shellfish producers, scientists, and coastal states are ganging up on ocean acidification (PDF)